Shapes of s and p orbitals
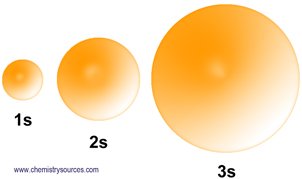
s orbital can have only one possible orientation (as it’s m quantum is zero), it is spherical in shape i.e., in an s orbital the electron distribution is symmetrical around the nucleus in all directions. However the size of s orbital depends on principal quantum number. Large n means large size. Thus 3s orbital is larger than 2s orbital.
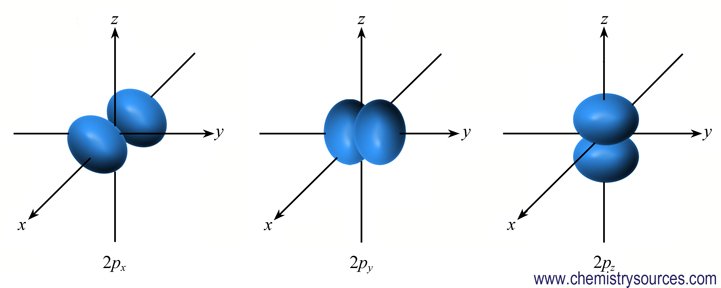
There are three p orbitals (px, py, pz) as the values of m are three i.e., -1, 0, +1 and all are of equal energy. They are dumb-bell shaped and oriented along three axis x, y and z respectively.