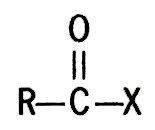One of a large group of organic substances possessing the halocarbonyl group,
in which X stands for fluorine, chlorine, bromine, or iodine. The terms acyl and aroyl halides refer to aliphatic or aromatic derivatives, respectively.
The great inherent reactivity of acid halides precludes their free existence in nature; all are made by synthetic processes. In general, acid halides have low melting and boiling points and show little tendency toward molecular association. With the exception of the formyl halides (which do not exist), the lower members are pungent, corrosive, lacrimatory liquids that fume in moist air. The higher members are low-melting solids.
Acid chlorides are prepared by replacement of carboxylic hydroxyl of organic acids by treatment with phosphorus trichloride, phosphorus pentachloride, or thionyl chloride.
Although acid bromides may be prepared by these methods, acid iodides are best prepared from the acid chloride treatment with either CaI2 or HI, and acid fluorides from the acid chloride by interaction with HF or antimony fluoride.
The reactivity of acid halides centers upon the halocarbonyl group, resulting in substitution of the halogen by appropriate structures. Thus with substances containing active hydrogen atoms (for example, water, primary and secondary alcohols, ammonia, and primary and secondary amines), hydrogen chloride is formed together with acids, esters, amides, and N-substituted amides, respectively.