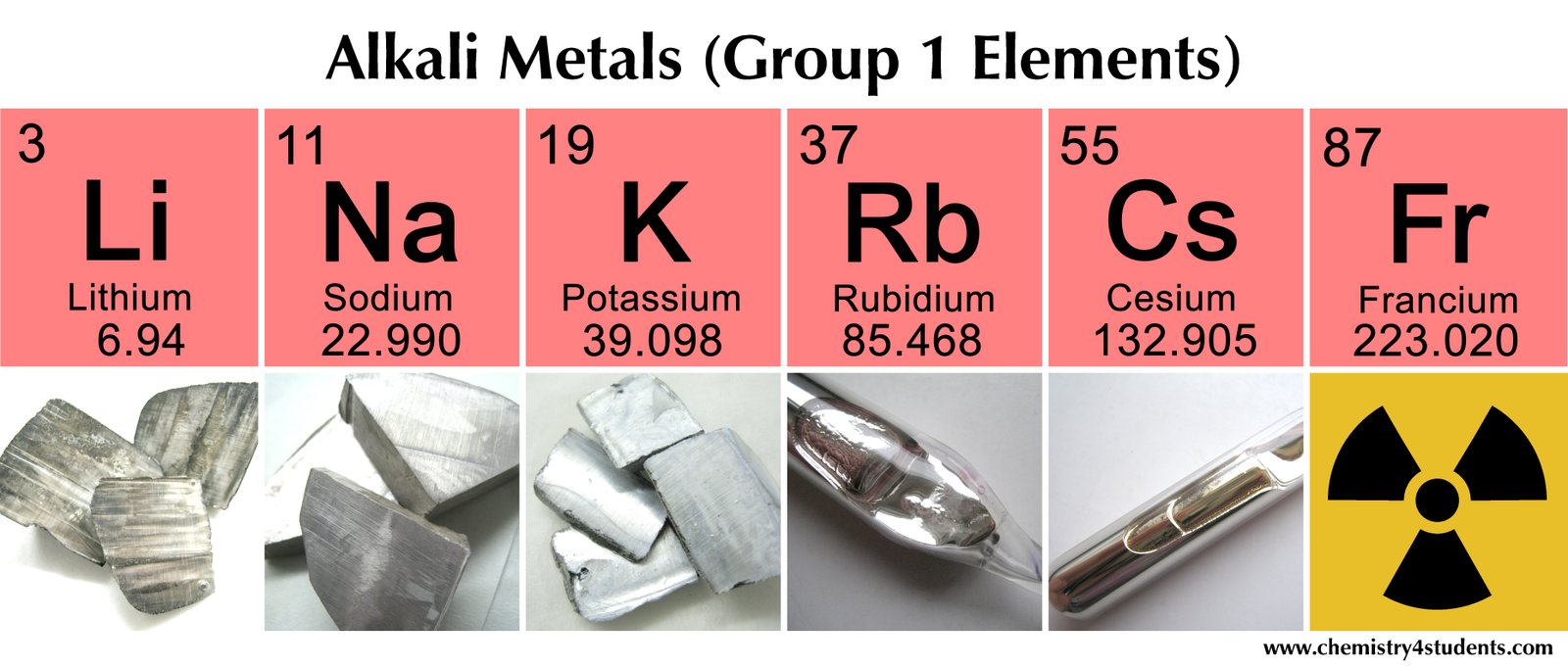
alkali metals (group 1 elements)
A group of soft reactive metals, each represent in the start of a new period in the periodic table and having an electronic configuration consisting of a rare-gas structure plus one outer electron. The alkali metals are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They formerly were classified in subgroup IA of the periodic tabl
Specifications of alkali metals (group 1 elements)
The elements all easily form positive ions M+ and consequently are highly reactive (particularly with any substrate that is oxidizing). As the group is descended there is a gradual decrease in ionization potential and an increase in the size of the atoms; the group shows several smooth trends which follow from this. For example, lithium reacts in a fairly controlled way with water, sodium ignites, and potassium explodes.
There is a general decrease in the following: melting points, heats of sublimation, lattice energy of salts, hydration energy of M+, ease of decomposition of nitrates and carbonates, and heat of formation of the ‘-ide’ compounds (fluoride, hydride, oxide, carbide, chloride). Lithium has the smallest ion and therefore the highest charge/size ratio and is polarizing with a tendency towards covalent character in its bonding; the remaining elements form typical ionic compounds in which ionization, M+X–, is regarded as complete.
The slightly anomalous position of lithium is illustrated by the similarity of its chemistry to that of magnesium. For example, lithium hydroxide is much less soluble than the hydroxides of the other group 1 elements; lithium perchlorate is soluble in several organic solvents. Because of the higher lattice energies associated with smaller ions lithium hydride and nitride are fairly stable compared to NaH, which decomposes at 345°C. Na2N, K3N etc., are not obtained pure and decompose below room temperature.
The oxides also display the trend in properties as lithium forms M2O with only traces of M2O2, sodium forms M2O2 and at high temperatures and pressures MO2, potassium, rubidium, and cesium form M2O2 if oxygen is restricted but MO2 if burnt in air. Hydrolysis of the oxides or direct reaction of the metal with water leads to the formation of the hydroxide ion.
Salts of the bases MOH are known for all acids and these are generally white crystalline solids. The ions M+ are hydrated in water and remain unchanged in most reactions of alkali metal salts. Because of the ease of formation of the ions M+ there are very few coordination compounds of the type MLn+ apart from solvated species of very low correlation times.
The group 1 elements form a variety of organometallic compounds; the bonding in lithium alkyls and aryls is essentially covalent but the heavier elements form ionic compounds. Organo-alkali metal compounds– particularly the lithium compounds– are widely used in synthetic organic chemistry.
Francium is formed only by radioactive decay and in nuclear reactions; all the isotopes of francium have short half-lives, the longest of which is 21 minutes (francium- 223). The few chemical studies which have been carried out indicate that it would have similar properties to those of the other alkali metals.
بوسترات (لوحات) كيميائية بدقة عالية (أكثر من 25 لوحة) من تصميم الأستاذ أكرم أمير العلي
بعض التطبيقات الكيميائية من تصميم الأستاذ أكرم امير العلي متوفر للجوالات التي تعمل بنظام أندرويد android على سوق جوجل بلاي google play
1 – تطبيق ملصقات كيميائية: ملصقات بتصميم جميل جدا للكواشف و الأدلة و الزجاجيات المستخدمة في المختبر و كذلك ملصقات و بطاقات لخزانات حفظ المواد و الأدوات الزجاجية .
https://play.google.com/store/apps/details?id=com.akramir
2 – إذا كنت تواجه صعوبة في تحضير المحاليل الكيميائية الأكثر شيوعا في مختبرات الكيمياء و الاحياء، فهذا التطبيق سوف يساعدك كثيرا في تحضير المحاليل :
 مصادر الكيمياء موقع لتعليم الكيمياء بصورة مبسطة
مصادر الكيمياء موقع لتعليم الكيمياء بصورة مبسطة