Hydrogen bond: It is a bond formed between a hydrogen atom attached to a highly electrovalent atom (F,O,N) and another highly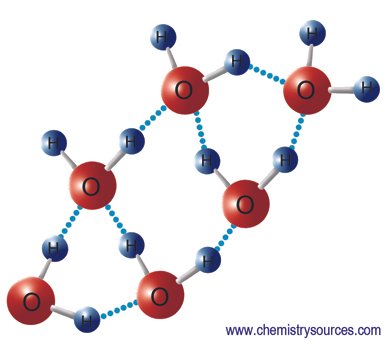
Condition for hydrogen bonding :
(i) The electronegative atom attached to the hydrogen should be small.
(ii) The electronegativity of the atoms to which hydrogen .atom is attached should be high.
Hydrogen bond in H-F molecule. H2 atom contains one proton and one electron. When the hydrogen atom is attached to F (strongly electronegative atom) the electrons . of the covalent bond are displaced towards F. It results a fractional . positive charge on the hydrogen atom and a fractional negative charge on electronegative atom F.
The positively charged hydrogen atom attracts and is attracted by ·the negative end of the, other molecule. Thus two or more molecules may associate together to form clusters of molecules.
Characteristics of Hydrogen bond :
(i) Hydrogen bond is electrostatic in nature and thus compound are strongly polar.
(ii) Hydrogen bond” is only 5to 10% (5 K cals/mole} as strong as that an ordinary covalent bond but is often stronger than other inter molecular forces of the dipole and Van der Waall’s forces.
(iii) The strength of hydrogen bond tends to increase as the electronegativity of the element increases.
(iv) Hydrogen bond. effects ‘all physico-chemical properties such as melting points , boiling points, solubility and spectra etc.